It is very common in the pharmaceutical and medical devices industry to convene expert meetings to arrive at and publish an expert consensus around the diagnosis and/or treatment of a disease condition. The objective is to create greater awareness among the medical fraternity about the condition and expert guidance in managing it. Another objective is to minimize the divergent practices in managing a disease condition and guide clinicians in achieving optimal outcomes that balance the risks, benefits, and costs. Unfortunately, a large proportion of such publications are rejected by leading journals due to methodological flaws in the consensus process. Not succeeding in having a publication or publishing such consensus statements in journals with low-impact factor fails to have the desired result as the reach of such journals is very poor.
While most consensus-based manuscripts claim that the consensus was arrived at using a ‘modified’ Delphi process, there is no standard definition of a ‘modified’ Delphi process. Hence, the outcomes of such initiatives are often not considered robust, and leading journals usually reject such publications.
The Delphi process is a structured process, in which modifications should not compromise the basic flow and principles. The process is briefly explained below and should be adhered to in order to achieve a robust consensus that can make an impact on the literature and the clinical fraternity.
Some mandatory rules in the Delphi process are as below:
- The voting should always be anonymous
- The Delphi process is iterative, involving multiple rounds of voting and discussions
- There should be a pre-determined statistical analysis plan and a definition of the level of agreement required to state that consensus has been reached.
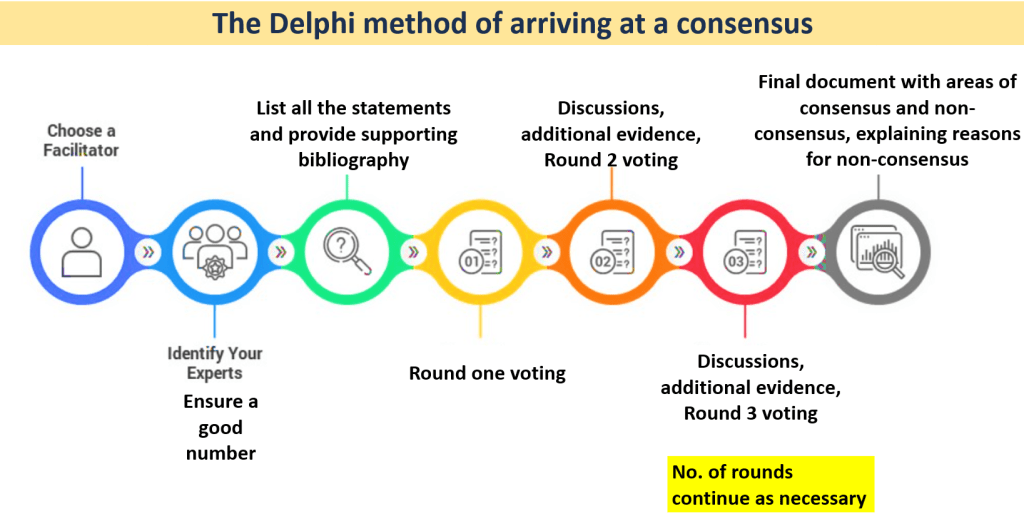
Here are the steps of the Delphi process in sequential order:
- Select a group of experts to participate in the process. It is usually considered inadequate to have fewer than 10 experts on the panel. A larger number also ensures that the outcomes are not compromised if some people do not vote in subsequent rounds. Generally, a number close of 30-50 is considered optimum in concluding rounds for a homogenous Delphi.
- Identify a facilitator. The facilitator coordinates the process and is responsible for sending out questionnaires and moderating the discussions.
- A first round of meeting of the panel to agree on the scope of the consensus and the items/questions that the consensus will aim to address.
- A list of statements is sent out to the panel members along with bibliography in support of the statements (the bibliography at this stage is optional and can also be shared along with results of the voting). Panelists vote for their level of agreement with each question (or statement) on a Likert scale.
- A second meeting is convened after the results of the voting are shared. At this meeting, panel members can present their views in favor of or against any statement along with published evidence and their clinical practice insights. The questions are modified by the convenor if necessary, based on previous responses and suggestions from panelists.
- The questions are sent for voting again to see if a consensus is achieved on statements that did not achieve consensus earlier or if there is a change in voting on statements that achieved consensus earlier. Subsequent meetings and rounds of voting can be continued until panel members feel that there is no likelihood of further change in the voting outcomes. Having 3 rounds of voting is the commonest norm.
It is important to note that for a robust outcome, the response rate of each Delphi round should not fall below 70% of the panel size. No subsequent rounds should be conducted if the response rate of the previous round is low. Hence, it helps to have a panel that is not very small to begin with.
The above Delphi process if followed well is likely to achieve impactful outcomes and a greater acceptance by journals that have a high readership.