Newsletters are a very useful tool for scientific communication if the topics chosen are such that they evoke interest. However, with most pharma and medical devices sales rep leaving a large amount of literature with the HCPs, how do you stand out?
Here are some key points to keep in mind.
Medical affairs and marketing need to brainstorm and come up with topics relevant to a clinician, such that reading the content makes him/her feel like exploring the topic further or giving a thought to implementing the concept in clinical practice. Usually, scientific newsletters should aim at sharing newer concepts and data related to a product (not a brand) or disease area. The objective should be to build HCP relationships through scientific engagement. Stay away from trying to sell your brand through a scientific newsletter.
It is important to understand that concepts are not built quickly with a newsletter or two. Hence, it is good to plan a series that will run over a period of some months with a new edition at fortnightly or monthly intervals. Monthly is better because it gives time for the message to sink in. Before you begin, write down what is the big message that you want to leave the reader with, at the end of the series. What are the submessages that can lead to the big message? The big message you want to convey could be chosen based on market dynamics or new research that is likely to reshape clinical practice related to a disease area. Once again, customer insights are key, before planning any customer communication. Hence, keeping in touch with your customers and meeting them on a regular basis is paramount.
As an example, maybe you are trying to increase the usage of a drug or medical device in clinical practice. Your big message might be that using the drug or the device leads to better outcomes overall. There might be studies about the same. If you plan a series of newsletters each talking about a different study showing better overall outcomes, the reader will lose interest after the first one or two editions. How do you maintain the interest? Maybe break it down into sub-messages and focus on one sub-message in each subsequent edition.
Here is an example- let us say your product is a drug used in heart failure. You could plan a series of newsletters based on its benefits in different patients. Each edition could cover only 1 benefit e.g., decrease in the number of hospitalizations, improvement in exercise parameters, improved quality of life etc. Let us say we choose the topic ‘decrease in the number of hospitalizations.’ You could pick studies where this benefit has been proven. However, simply compiling these studies together as a newsletter is not likely to interest the readers. You need to create a well-flowing story in which the data of the studies fits in as part of the narrative. You could end the newsletter with a summary of patient profiles in which this benefit has been found to be the best. It is a good idea to create a list of topics for the newsletter series, planning what message each edition will convey, even before you begin working on the first edition. Avoid topics such as data of the [drug name] in patients with HFrEF (heart failure with reduced ejection fraction) about which a clinician is likely to know the data on the tips of his/her fingers already, as that will not create interest.
Another idea for a newsletter series is building a new concept that could lead to change in or modify treatment practice. Let us look at an example. Maybe you are trying to establish the concept of a single long stent for long coronary lesions instead of the use of 2 stents to cover the long lesion. Some of the topics for this series could be – 1) the epidemiology about long coronary lesions comparing data from various geographies and the reason for the difference, as well as long-term outcomes of long lesions. 2) Evolution of the 2 stent to 1 stent concept. 3) Controversies about the 1-stent vs 2-stent approach 4) Data from clinical studies vs real-world data with stents 5) Predictors of poor outcomes with 2 stents 5) Data of single long stent from trials as well as real-world data 6) Long term outcomes of 2 stents and single stent.
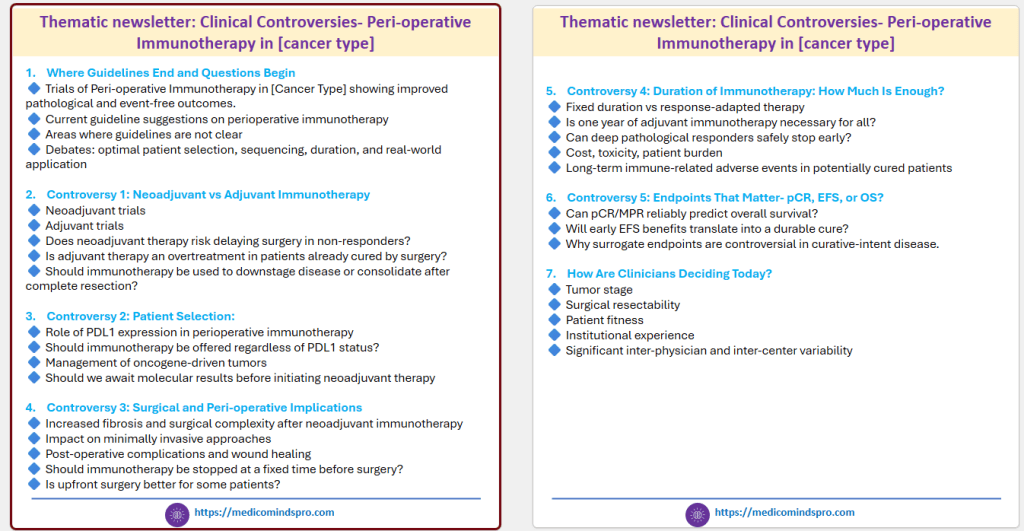
An interesting type of newsletter could be a thematic newsletter. These could focus on addressing clinical dilemmas, practice variation, and unanswered questions. Example, a multi-edition thematic newsletter series titled:
“Clinical Controversies: Peri-operative Immunotherapy in [Cancer Type]”. Each edition (5-6 pages) can focus on one controversy, with published evidence. Such newsletters are relevant, practical, and worthy of clinicians’ time. Here is an example of such a newsletter covering 7 editions.
A very routine type of newsletter, which is not directly related to a product or concept, could be simply a compilation of the latest published studies related to a therapy area. However, remember that this type of newsletter is no longer considered very novel. A sample is attached below. Nevertheless, to attract interest, the key to success is picking studies that are interesting for the clinician. For e.g., yet another study showing the efficacy of SGLT2 inhibitors in cardioprotection may not be very interesting as this benefit has been proven beyond doubt and accepted. However, something like the benefits of the drug post-acute myocardial infarction might be interesting. However, this type of newsletter demands agility. If a study published in January is shared in April, it might be too old already and might have been read. It needs team coordination to pick the latest studies and ensure that too much time is not spent in creating, designing, printing, and dispatching the newsletter. Studies published in January should reach the reader no later than mid-February and earlier if possible. Only then will the newsletter create interest, and readers will look forward to your editions. If you look at the sample below, it is now too outdated as most cardiologists would be aware of this data although these studies were very new when I created the newsletter.
What should be length of the newsletter? While there is no universal norm, it can be as small as 2 A4 pages or as long as 8-10 pages. It completely depends on the topics and the data available. However, in today’s times, less is more. Perhaps, 4 pages might be ideal.
Next comes the design. For your scientific newsletter, use as many infographics as possible and lay it out in a way such that each section stands out visually. The headline has to be eye-catching at the same time scientifically correct (i.e., not making false claims). Your key message has to be in the beginning or at the end of the conclusion so that the context of the communication is not lost.
Finally, the end of the newsletter can have an interesting hook as well. It could be a teaser about the next edition or a teaser of an upcoming launch or campaign, or even a real-world or published case related to the topic of the newsletter. The case should be covered in not more than 8 to 10 sentences. If it is a real-world case, the HCP who has contributed to the case needs to be acknowledged with his/her consent. If none of these ideas seem interesting for an interesting end, look for an interesting ‘did-you-know’ medical trivia that HCPs will likely enjoy knowing about.

