Importance of advisory board meetings
As all of us in the healthcare industry know, an advisory board is a group of experts invited to a meeting by a company (pharma or medical devices) to seek their professional advice and insights. Usually, insights are sought about a disease, its management, and the experts’ opinions on studies of a new drug (just launched or to be launched). The insights of these experts are much sought after as it is practical, real-life knowledge gained over years of experience- something that cannot be found in medical textbooks.
These meetings are extremely important for the company organizing them as their marketing strategies depend on these insights. These also help the organization plan other initiatives like consensus or expert opinion publications, patient education, patient support programs, training classes for paramedics, and others. While the teams in charge of these meetings are the medical affairs and marketing teams, the report of an advisory board is an EXTREMELY CRUCIAL document. It is often sent to the senior management of the organization as the strategies based on these might require their sign-off, and of course also to the experts who were a part of the meeting. Hence, it is important that the report is neither too concise nor too exhaustive, yet incisive enough.
Typical flow of an advisory board meeting
Usually, the meeting has 3-4 sessions. Each session has a presentation followed by a discussion among experts. There is a moderator who might be from among the experts or a medical affairs professional from the organizing company takes up that role- the former is more common. The presentations might be made by the medical affairs team and/or by the experts from the team. Of course, all of this is planned months in advance, including the content to be presented. Organizing an advisory board meeting is a topic in itself that can be discussed separately.
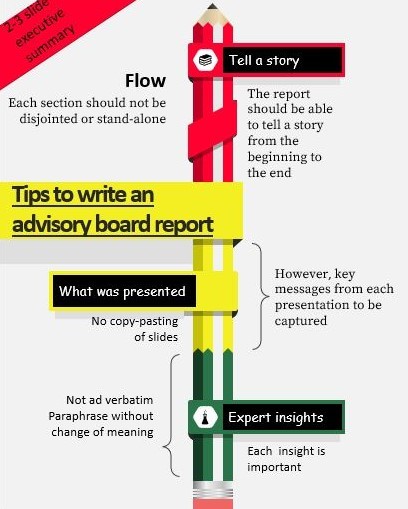
How to write the report
- The report might be in a word or PowerPoint format as required by the organizers.
- Like any other professional report, it should be able to tell a story from the beginning to the end; there needs to be a flow, and each section should not be disjointed or be a stand-alone section.
- It should begin with the basics like the title, attendees, absentees, objectives, and agenda.
- This should be followed by an executive summary that conveys the key highlights of the meeting in about 3 slides- this will be a succinct summary of the sections below.
- How to capture what was presented at the meeting is the most tricky part. The experts obviously would not want to spend time reading everything that was presented at the meeting, all over again. At the same time, the senior management needs to be informed about what was presented not only to provide context to the insights but also to complete the story and yet keep the report concise. Instead of copy-pasting the slides presented, what needs to be incorporated are the key messages from each presentation that the speaker would have highlighted while presenting. To capture this effectively, it is very important to be 100% attentive during the sessions and take notes. In fact, it is this part of the report that most writers struggle with, which not only fails to present the entire report as a story to the management but might also upset the experts.
- The most important part of the report is of course the insights from the experts. Every word spoken is critical but at the same time, they should not be put down ad verbatim. While not missing anything spoken, it is important to paraphrase them without changing the meaning whatsoever.
- The order of the sections should be the same as that in the meeting.
- Once written, make sure you read the report from beginning to end and check that it conveys a meaningful story that automatically leads towards the next steps that should be thought of.
- Check whether the story is good enough for the management to frame a strategy, is it good enough to be converted into a consensus manuscript or an expert opinion publication?
- To conclude, an advisory board report should appear as a highly professional document worthy of being shared with the seniormost level of the management in the organization and also make the experts feel that their insights have been captured very well and it has been worth the time they have spent preparing for the meeting as well as participating in a meeting.
- In fact, if written well, such reports can be circulated as booklets or white papers to other HCPs as well.