Owning the Grey Zone Beyond Guidelines is a very powerful positioning strategy, especially for drugs with limited head-to-head data.
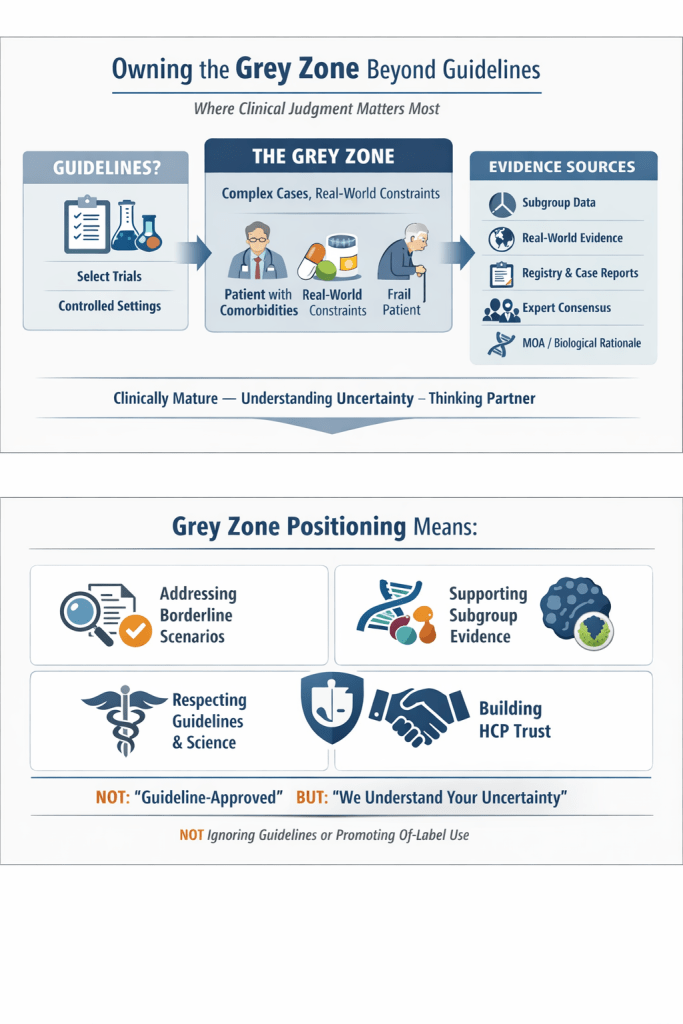
Guidelines are essential, but every day, clinicians need to make decisions where evidence is incomplete, and cases are complex. This is the grey zone of medicine where clinical judgment matters most.
Evidence considered in guideline recommendations is often based on select trial populations, fixed inclusion criteria, and controlled settings. Many drugs are not guideline-endorsed in specific situations, such as borderline cases, patients with comorbidities, and real-world constraints (cost, access, skill, anatomy, adherence).
Yet evidence in the form of specific subgroup data in clinical trials, real-world evidence, registries, case reports, expert consensus, or the mechanism of action might show the likely benefit or safety of a drug in specific patient populations.
One of the most interesting examples is carboplatin in oncology. If you strictly follow guidelines, carboplatin has clearly defined roles. But in real clinical practice, Carboplatin shows up in treatment decisions far beyond where guidelines explicitly place it. Compared to cisplatin, carboplatin has a more predictable toxicity profile, is easier to administer, and is often better tolerated in frail or comorbid patients. Guidelines may prefer cisplatin in many settings. But at the bedside, clinicians often ask a different question: “What is the best balance between efficacy and tolerability for this patient?” That question is rarely answered by a guideline table.
Another example is immunotherapy. Immunotherapy guidelines are based on narrowly defined trial populations and are indication- and biomarker-driven. But real immunotherapy practice involves: toxicities that are non-linear, biomarkers that are imperfect, patients who are heterogeneous, and responses that are unpredictable. Yet in practice, clinicians use them in molecular subgroups not yet guideline-endorsed, especially where there is a strong biological rationale and early evidence.
Guideline-based positioning says “We are guideline-approved.”
Grey-zone positioning says “We understand your uncertainty”. Owning the Grey Zone DOES NOT MEAN ignoring guidelines, promoting off-label use, or positioning against evidence. Hence, the language to be used is very important.
One way of owning the grey zone could be: ABC Clinical scenarios where guideline recommendations are absent, conditional, or difficult to apply, 12345 evidence shows that drug X could be a clinically reliable option because…(state scientific rationale)
This type of positioning differentiates the brand from competitors and leads to better brand recall and preference. It positions the brand as:
- Clinically mature
- Understanding HCPs’ real-world challenges
- Intellectually honest
- A thinking partner, not a sales voice
To own the Grey Zone, you need a strong medical affairs partner who can master the evidence and present data as clinical actions. Trust MedicoMindsPro as your medical affairs partner for strategic positioning of your brand.