International guidelines on atrial fibrillation do not recommend a specific DOAC and suggest that the choice should be tailored to each patient’s renal function, bleeding risk, GI tolerance, lifestyle, and cost considerations. Each molecule has its own benefits and drawbacks.
My client had a brand of rivaroxaban, which was competing against apixaban. The perception created in the market by the apixaban brands was that apixaban is superior to rivaroxaban because it has a lower incidence of GI bleed and does not need to be taken with food. Hence, it should be preferred in the elderly (the main target segment for atrial fibrillation).
My client’s brand was struggling despite a once daily dosing, due to the strong messaging by competitors about the above 2 benefits of apixaban, although the dose of apixaban is twice daily. The challenge was to re-position the brand such that the benefits of rivaroxaban can overpower the created negative perception. We were clear that we did not want to talk against another molecule but rather bring out the strength of rivaroxaban.
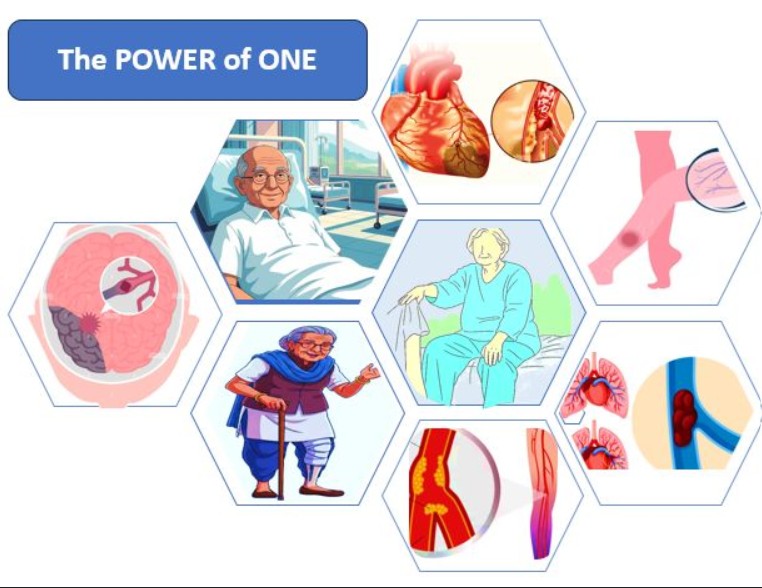
As usual, my approach was to look at science first and then think of the possible messaging. Looking at the data and studies, it was found that rivaroxaban was approved for certain indications for which apixaban was not. Elderly patients were more vulnerable to these specific conditions. All that was required was to tie the strings together.
The simple message was – ‘The power of ONE’. One drug to cover all the related risks in the elderly and in a single dose. We now knew what brief to give to the creative team – to create the persona of a patient who is at risk of all the conditions that rivaroxaban is useful for, justifying the power of ONE.
Recreating a brand positioning through a patient persona: an interesting case of a rivaroxaban brand